Ask AI on The Internet
Question: write a total of 350 words about how diamonds are made, why white phosphorous combusts in air, and about neutralization reactions
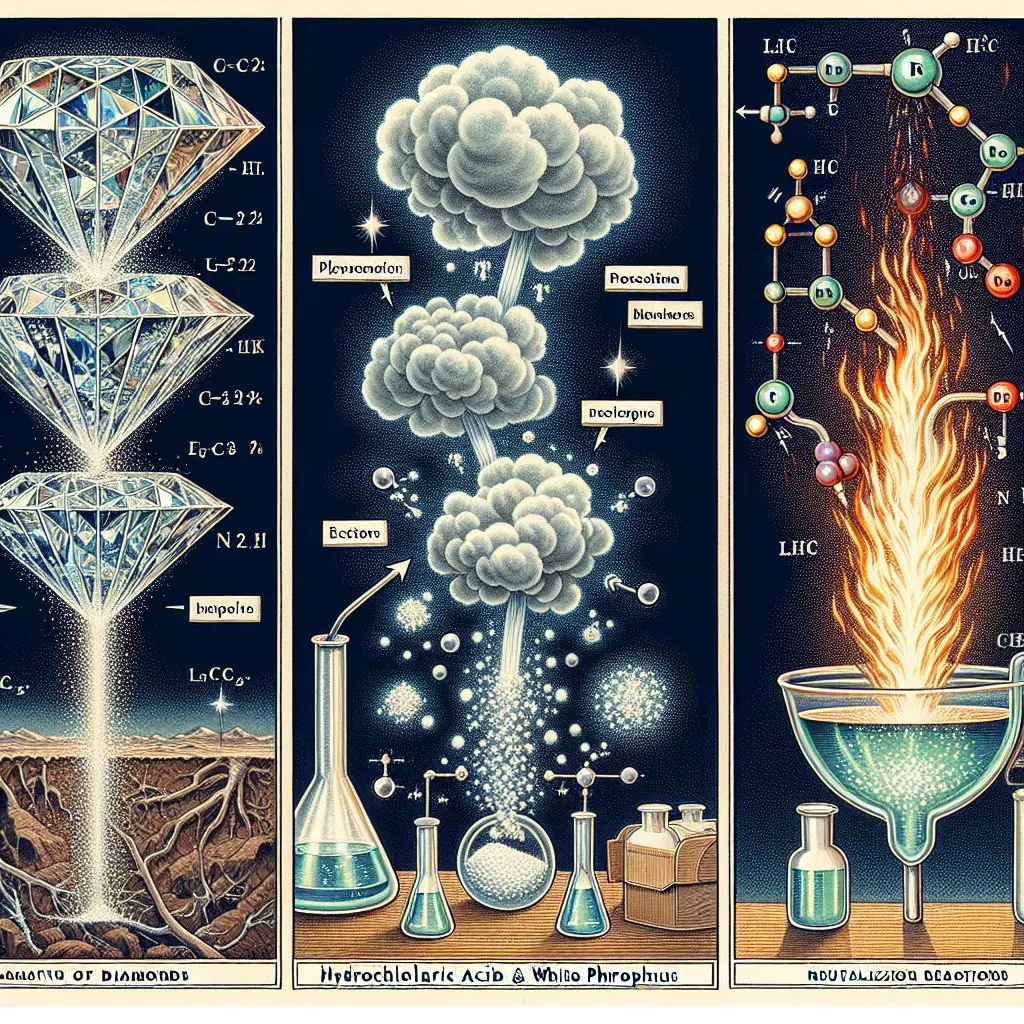
### How Diamonds are Made Diamonds are formed deep within the Earth's mantle under extreme pressure and high temperatures. These conditions are typically found at depths of around 150 to 200 kilometers below the Earth's surface. The carbon atoms in the mantle bond together in a crystal lattice structure, creating diamonds. Over millions of years, volcanic eruptions can bring these diamonds closer to the Earth's surface, where they can be mined. Aside from natural processes, diamonds can also be created synthetically in laboratories through high-pressure high-temperature (HPHT) or chemical vapor deposition (CVD) methods. HPHT mimics the natural formation process by applying high pressure and temperature to carbon sources, whereas CVD involves breaking down gases like methane into carbon atoms that then crystallize into diamond structures on a substrate. ### Why White Phosphorous Combusts in Air White phosphorus is highly reactive due to its molecular structure, which consists of P4 tetrahedra where each phosphorus atom is connected to three other phosphorus atoms. These atoms are held together with significant strain, making the molecule energetically unstable. When exposed to air, the high reactivity and the presence of oxygen lead to oxidation of white phosphorus, causing a vigorous exothermic reaction. This reaction releases enough heat to ignite white phosphorus, causing it to combust spontaneously at around 30°C (86°F). The resultant product is phosphorus pentoxide (P4O10), which is seen as white smoke. The high reactivity and spontaneous combustion in air render white phosphorus dangerous and useful for various applications, including military and industrial uses. ### Neutralization Reactions Neutralization reactions are chemical reactions wherein an acid and a base react to form water and a salt. These reactions are essential in chemistry and various industrial and biological processes. The general form of a neutralization reaction is: \[ \text{Acid} + \text{Base} \rightarrow \text{Salt} + \text{Water} \] For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are sodium chloride (NaCl) and water (H2O): \[ \text{HCl} (aq) + \text{NaOH} (aq) \rightarrow \text{NaCl} (aq) + \text{H_2O} (l) \] The acid donates a proton (H+) and the base donates a hydroxide ion (OH-), which combine to form water. Neutralization reactions are frequently used to adjust the pH of solutions, treat wastewater, and in antacid medications to relieve heartburn. The resulting salt's properties depend on the specific acid and base used, influencing its uses and applications in various industries.
Think your answer is better than the AI's? Post it below.
Other Most Relevant Posts:
 Ask AI: 1000 WORDS INTRODUCTION TO FIRST ORDER REACTION:
DECOMPOSITION OF HYDROGEN PEROXIDE. INTEXT REFERENCE
Ask AI: 1000 WORDS INTRODUCTION TO FIRST ORDER REACTION:
DECOMPOSITION OF HYDROGEN PEROXIDE. INTEXT REFERENCE
 Fluoro Alcohols and Poly Fluoro Alcohols: The Basics -> Basics of Fluoro Alcohols
P Phase Compounds: Tough Cookies -> Toughness of P Phase Compounds
P Phase Products Through the Years -> Evolut
Fluoro Alcohols and Poly Fluoro Alcohols: The Basics -> Basics of Fluoro Alcohols
P Phase Compounds: Tough Cookies -> Toughness of P Phase Compounds
P Phase Products Through the Years -> Evolut
Question Tags
If you want your question answered by an AI, click here.



Post your own comment: