Ask AI on The Internet
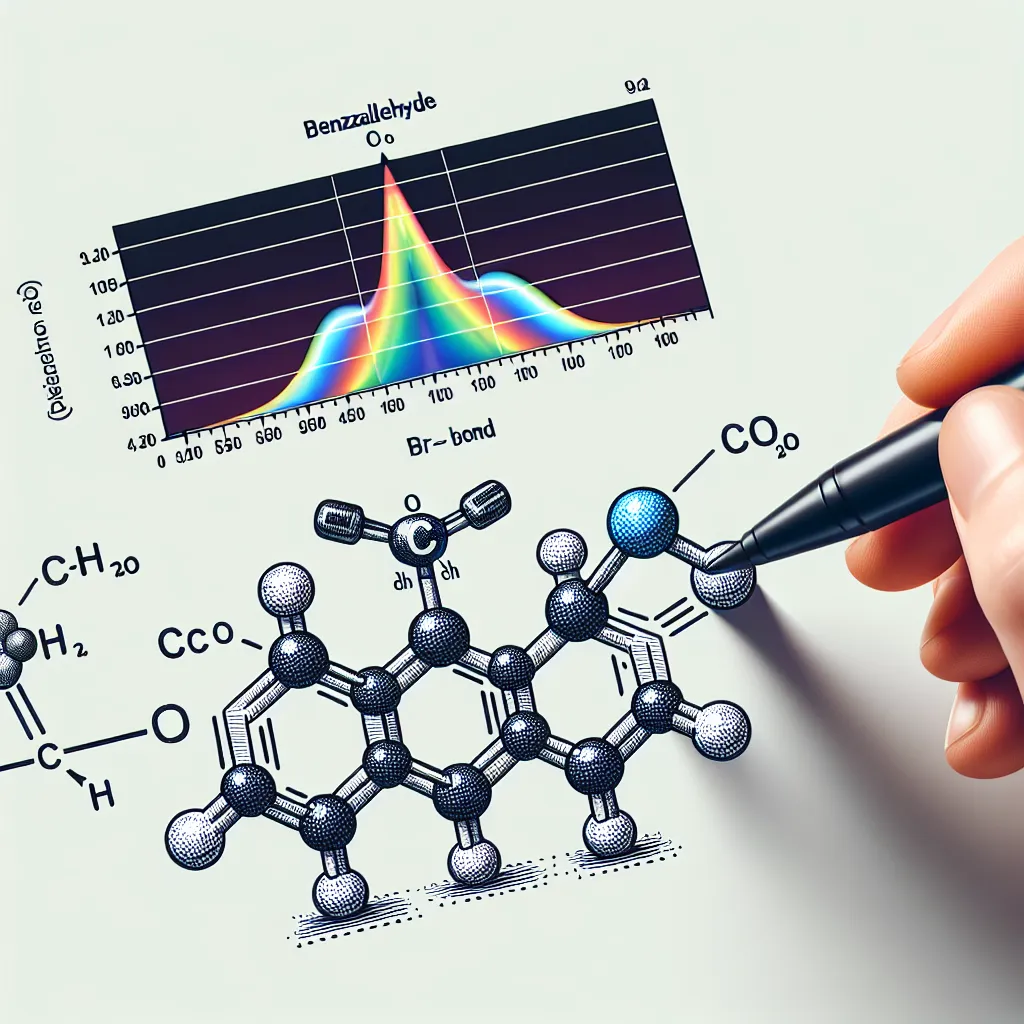
To identify the carbonyl (C=O) stretch in the IR spectrum of benzaldehyde, you should look for a strong, sharp absorption band. For benzaldehyde, this band typically appears in the range of 1700-1725 cm⁻¹. Here are the steps to identify it: 1. **Obtain the IR Spectrum**: Ensure you have the IR spectrum of benzaldehyde. 2. **Locate the Characteristic Peak**: Look for a prominent peak within the 1700-1725 cm⁻¹ range. 3. **Interpret the Peak**: Verify that this peak represents the C=O stretching vibration, which is generally very strong and sharp due to the polar nature of the carbon-oxygen double bond. In most cases, for benzaldehyde specifically, you will likely see the carbonyl stretch closer to 1700 cm⁻¹. Keep in mind that the exact position can be influenced by conjugation and substituents, but for benzaldehyde, it will predominantly reside in this general region.
Think your answer is better than the AI's? Post it below.
Question Tags
If you want your question answered by an AI, click here.

Post your own comment: